Coulomb's Law and Electric Force
Coulomb's Law is the fundamental equation that describes the electric force between two point charges. It was discovered by Charles-Augustin de Coulomb in 1785 and forms the foundation of electrostatics.
What is Coulomb's Law?
Coulomb's Law states that the magnitude of the electric force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
- F: Electric force (N)
- k: Coulomb's constant = 8.99 × 10⁹ N⋅m²/C²
- q₁, q₂: Charges of the two objects (C)
- r: Distance between the charges (m)
Coulomb's constant is related to the permittivity of free space (ε₀) by the equation above. The permittivity of free space is ε₀ = 8.85 × 10⁻¹² C²/N⋅m². We will talk more about ε when we talk about capacitors.
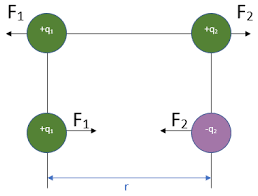
Law of Charges
The fundamental law of electric charges governs all electrostatic interactions:
- Like charges repel: Two positive charges or two negative charges will push each other apart.
- Opposite charges attract: A positive charge and a negative charge will pull each other together.
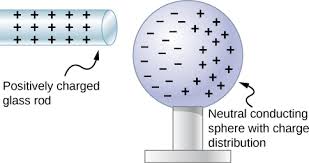
- Neutral objects: Objects with no net charge experience no electrostatic force. However, if the object is polarized, it will experience a force (common in conductors).
- Force strength: The magnitude of the force depends on the amount of charge and the distance between charges. The magnitude of the force on each object is the same due to Newton's 3rd law
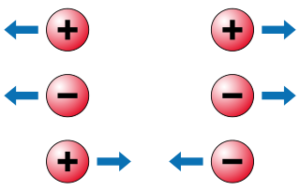
Vector Form of Coulomb's Law
The vector form includes the direction of the force. The unit vector \(\hat{r}_{12}\) points from charge 1 toward charge 2. This determines whether the force is attractive or repulsive.
Key Properties of Coulomb's Law
- Inverse square law: Force decreases as 1/r²
- Superposition principle: Total force is the vector sum of individual forces
- Conservative force: Work done is path-independent
- Long-range force: Acts over large distances
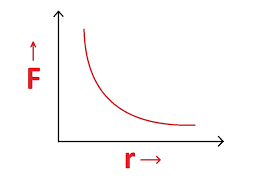
Comparing Electric and Gravitational Forces
Both forces follow inverse square laws, but electric forces can be attractive or repulsive, while gravitational forces are always attractive.