Electromotive Force (EMF) and Batteries
Electromotive force (EMF) is the voltage generated by a source of electrical energy, such as a battery. While EMF is measured in volts, it represents the energy per unit charge that the source can provide, not the actual voltage across its terminals when connected to a circuit.
What is EMF?
Electromotive force (EMF) is the voltage that a battery would provide if no current were flowing through it. It's the "ideal" voltage of the battery before any current is drawn.
- Symbol: \(\mathcal{E}\)
- Units: Volts (V)
- Simple definition: The voltage a battery provides when not connected to anything
- Relationship to voltage: EMF is the maximum voltage the battery can provide
EMF vs Terminal Voltage
The key distinction in AP Physics C is between EMF and terminal voltage:
- EMF (\(\mathcal{E}\)): The voltage the battery would provide in an open circuit (no current)
- Terminal Voltage (V): The actual voltage across the battery terminals when current is flowing
- Relationship: Terminal voltage is always less than or equal to EMF
Memory Trick: EMF vs Terminal Voltage
Think of EMF as the "advertised" voltage and terminal voltage as the "actual" voltage:
- EMF (ε): "This battery can provide 12V" (when not connected)
- Terminal Voltage (V): "This battery actually provides 11.5V" (when connected to a circuit)
- The difference: Internal resistance causes voltage drop
This helps you remember that EMF is the ideal voltage, while terminal voltage is the real voltage under load.
Battery as a Voltage Source
A battery converts chemical energy into electrical energy, creating a voltage difference between its terminals. This voltage drives current through a circuit.
Battery Components
- Anode (negative terminal): Source of electrons
- Cathode (positive terminal): Destination for electrons
- Electrolyte: Chemical medium that allows ion flow
- Internal resistance: Resistance within the battery itself
Battery Operation
- Chemical reaction: Converts chemical energy to electrical energy
- Electron flow: Electrons move from anode to cathode through external circuit
- Ion flow: Ions move through electrolyte to maintain charge balance
- Voltage maintenance: Chemical reaction maintains voltage difference
Ideal vs Real Batteries
Ideal Battery
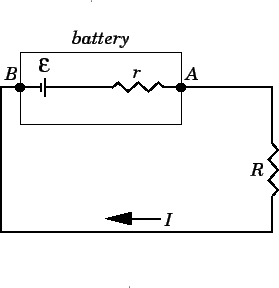
- Constant EMF: \(\mathcal{E}\) remains constant regardless of current
- No internal resistance: Terminal voltage equals EMF
- Infinite energy: Never runs out of charge
- Mathematical model: \(V = \mathcal{E}\)
Real Battery
- Internal resistance: Battery has internal resistance (covered in next topic)
- Voltage drop: Terminal voltage decreases with current
- Limited energy: Eventually runs out of charge
- Mathematical model: \(V = \mathcal{E} - Ir\) (covered in detail in next topic)
Battery Types and EMF Values
Common Battery Types
- Alkaline (AA/AAA): 1.5V EMF
- Lithium-ion: 3.7V EMF
- Lead-acid (car battery): 12.6V EMF
- Zinc-carbon: 1.5V EMF
- Nickel-cadmium: 1.2V EMF
Series and Parallel Batteries
- Series: EMFs add
- Parallel: Same EMF
Worked Examples
Interactive Battery Simulation
Explore how EMF affects battery behavior (internal resistance effects covered in next topic):